elisa test ebv|epstein barr ebv test : Brand manufacturer Bio-Rad offers complete microplate EIA kits for the detection of specific IgM and IgG antibodies to Epstein-Barr virus (EBV) antigens, aiding in determination of the stage of the EBV infection. Bio-Rad offers a comprehensive menu of EIA serology tests using standardized procedure and common reagents. The fundamental principles behind autoclave sterilization are pressure and temperature. Microorganisms have different heat resistance levels, and by subjecting them to high temperatures, their cellular structures break .
{plog:ftitle_list}
It is important to properly load and seal the sterilization pouches because it affects the sterilization time of the placed item. This is a complete guide for all healthcare professionals on how they can ensure safety by .
A rapid test for the detection of IgM and IgG Epstein-Barr nuclear antigen (EBNA-1) has been . Laboratory testing can help distinguish whether someone is susceptible to EBV .Serological tests for antibodies specific for Epstein-Barr virus (EBV) antigens are frequently .Bio-Rad offers complete microplate EIA kits for the detection of specific IgM and IgG antibodies to Epstein-Barr virus (EBV) antigens, aiding in determination of the stage of the EBV infection. Bio-Rad offers a comprehensive menu of EIA serology tests using standardized procedure and common reagents.
Anti EBV – VCA IgM, IgG, EBNA-1 IgG (skrining), IM test: ELISA Anti EBV heterofilné protilátky: IM test: latexová aglutinácia Anti EBV IgA, IgG, IgM (konfirmácia): Line Blot DNA EBV kvantitatívne: PCR. Význam stanovenia. Vírus Epstein-Barrovej (EBV = 4. ľudský herpetický vírus HHV4) je rozšírený po celom svete. Zdrojom EBV .Epstein-Barr virus EBNA-1 IgM ELISA (RE56261) FRANÇAIS Version 2013-05 1 / 6 1. BUT DU TEST Tests immuno-enzymatiques (barrettes microtitrées) pour le dosage qualitatif et quantitatif des anticorps IgM dirigés contre l’antigène nucléaire du virus Epstein-Barr (EBNA-1) dans le sérum et plasma humains. 2. SOMMAIRE ET INTRODUCTIONEpstein-Barr virus VCA IgM ELISA (RE56291) FRANÇAIS V2013-05 1 / 7 1. BUT DU TEST Tests immuno-enzymatiques (barrettes microtitrées) pour le dosage qualitatif et quantitatif des anticorps IgM contre l’antigène de la capside virale du virus Epstein-Barr (VCA) dans le sérum et plasma humains. 2. SOMMAIRE ET INTRODUCTIONEpstein-Barr virus (EBV), a pervasive virus that infects over 90% of the world’s population by adulthood, causes infectious mononucleosis (IM) in immunocompetent individuals and lymphoproliferative disease in immunocompromised patients. Most people are infected in childhood, at which time the infection is most often subclinical, but the virus remains as a p
Abcam's anti-Epstein Barr virus (EBV-VCA) IgM Human in vitro ELISA (Enzyme-Linked Immunosorbent Assay) kit is designed for the accurate qualitative measurement of IgM class antibodies against Epstein Barr virus in Human serum and plasma. A 96-well plate has been precoated with Epstein Barr virus antigens to bind cognate antibodies.In some of these cases, a test for Epstein-Barr virus antibodies may be useful. The most controversial use of EBV serology is in chronic fatigue syndrome, a complaint predominantly (but not exclusively) of young to middle-aged women, characterized by long persistent debilitating fatigue and a panoply of usually mild somatic complaints.Designed with the user in mind, the ZEUS ELISA™ menu of test systems for autoimmune and infectious diseases features common reagents and universal protocol that enable: Optimized workflow efficiency Simplified programming for automation Multi-batching of different ZEUS ELISA™ test systems on the same plate Faster integration into your laboratory with minimal . Background Nasopharyngeal carcinoma (NPC) is diagnosed relatively late and has a poor prognosis, requiring early detection to reduce the disease burden. This diagnostic test accuracy meta-analysis evaluated the serological diagnostic value of nine EBV-related IgA antibody panels (EBNA1-IgA, VCA-IgA, EA-IgA, Zta-IgA, EBNA1-IgA + VCA-IgA, VCA-IgA + .
The SERION ELISA classic Epstein-Barr Virus EA IgG, VCA IgG, IgM and EBNA1 IgG tests are quantitative and qualitative immunoassays for the detection of human antibodies in serum or plasma directed against components of the Epstein-Barr Virus (EBV). The SERION ELISA classic Epstein-Barr Virus EA IgG and SERION ELISA classic EBV VCA IgM are recommended for .EBV ELISA Kits Full Name. Epstein-Barr virus ELISA kits. Alternate Names for EBV ELISA Kits . EBV ELISA Kits Epstein-Barr virus ELISA Kits HHV4 ELISA Kits Human Herpesvirus 4 ELISA Kits Herpesviridae ELISA Kits Gammaherpesvirinae ELISA Kits Lymphocryptovirus ELISA Kits Human herpesvirus 4 (HHV-4) Epstein-Barr virus (EBV)-based serologic antibody testing has been found to be a feasible alternative for nasopharyngeal carcinoma (NPC) screening in endemic areas. The purpose of this study was to evaluate the performance of ELISA based on VCA IgA antibody, EA-IgA and Rta-IgG antibody specific to EBV in the diagnosis of NPC. A total of 2155 untreated .
Abstract. Epstein–Barr virus (EBV) is the causative agent of many diseases including infectious mononucleosis (IM), and it is associated with different subtypes of lymphoma, sarcoma and carcinoma such as Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, nasopharyngeal carcinoma, and gastric carcinoma.
monospot ebv test
The ELISA test kit provides semiquantitative in vitro determination of human antibodies of the immunoglobulin class IgM against Epstein-Barr virus early antigen diffuse (EBV-EA-D) in serum or plasma.Epstein-Barr virus EA IgG ELISA (RE56221) ENGLISH Version (01) 2013-05 4 / 6 11. TEST PROCEDURE 1. Pipette 100 µL of each Standard and diluted sample into the respective wells of the Microtiter Plate. In the qualitative test only Standard B is used. 2. Cove r plate with adhesive foil. Incubate 60 min at 18-25 °C. 3. Remove adhesive foil. Blood tests for Epstein-Barr virus (EBV) antibodies are used to help diagnose EBV infection, the most common cause of infectious mononucleosis (mono), if a person is symptomatic but has a negative mono test.. In pregnant women with symptoms of a viral illness, one or more EBV antibody tests may be ordered along with tests for cytomegalovirus (CMV), .
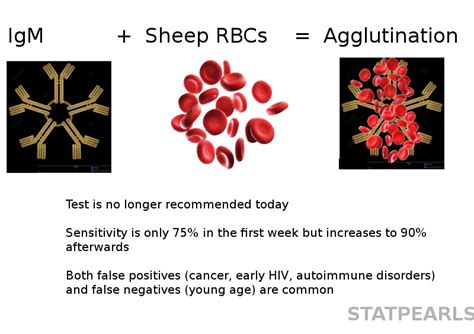
Serological tests for antibodies specific for Epstein-Barr virus (EBV) antigens are frequently used to define infection status and for the differential diagnosis of other pathogens responsible for mononucleosis syndrome. . Assessment of rapid ELISA test for detection of Epstein-Barr virus infection. J Clin Pathol. 1990;43:691–693. doi: 10. .Abcam’s anti-Epstein Barr virus (EBV-EBNA) IgG Human in vitro ELISA (Enzyme-Linked Immunosorbent Assay) kit is designed for the accurate qualitative measurement of IgG class antibodies against Epstein Barr virus nuclear antigen (EBNA) in Human serum and plasma.. A 96-well plate has been precoated with Epstein Barr virus antigens to bind cognate antibodies.Epstein Barr virus spada u rod Lymphocriptovirusa, a u porodicu Herpesviridae. Genom čini dvolančana DNK sa terminalnim ponavljajućim sekvencama. Omotač je troslojan. Replikuje se u jedru, ćeliju napušta lizom ili egzocitozom. . Dokazuje se serološkim reakcijama: Paul-Bunnell-ov test, ELISA test, indirektna imunoflorescencija. Lečenje.ZEUS ELISA EBV-VCA IgM Test System 2 (Rev. Date 12/13/2017) NOTES: 1. The following components are not Test System Lot Number dependent and may be used interchangeably within the ZEUS ELISA Test Systems: TMB, Stop Solution, and Wash Buffer. 2. Test System also contains a Component Label containing lot specific information inside the Test System .
ASI’s ELISA test kit is for the qualitative and semiquantitative detection of human IgG antibodies to Epstein-Barr virus nuclear antigen (EBNA) in human serum by enzyme immunoassay. The test may be used in conjunction with other EBV serologicals as .Imunoenzymatické soupravy ELISA-VIDITEST a MONO-VIDITEST jsou určeny pro detekci specifických IgA, IgG a IgM protilátek proti kapsidovému antigenu (VCA) viru Epstein-Barr (EBV) v lidském séru, plazmě i mozkomíšním moku a pro stanovení avidity a .The ZEUS ELISA EBV-EA IgG Test System is designed to detect IgG class antibodies to Epstein-Barr Virus Early Antigen in human sera. Creation of the sensitized wells of the plastic microwell strips occurred using passive adsorption with EBV-EA antigen. The test procedure involves three incubation steps:Two standard serological tests for diagnosing recent primary EBV infection, (i) the VCA IgG-EBNA antibody profile and (ii) detection of VCA IgM by indirect immunofluorescence (IF) were used as our reference assays for evaluating the new VCA IgM ELISA test (2, 8). Primary infection was defined as the presence of IgM antibody to EBV-VCA or the .
This family of EBV microplate ELISA test kits includes assays for EA-D IgG, VCA(p-18)IgG, VCA IgM, EBNA-1 IgG and EBNA-1 IgM. These assays can be used to determine an individual’s serologic EBV status and to aid in the diagnosis of .The ELISA test kit provides semiquantitative or quantitative in vitro determination of human antibodies of the immunoglobulin class IgG against Epstein-Barr virus early antigen diffuse (EBV-EA-D) in serum or plasma to support the diagnosis of infectious mononucleosis.The EBV-VCA IgM ELISA tests system is an enzyme linked immunosorbent assay (ELISA) for the detection of IgM class antibodies to EBV in humanserum or plasma. Diluted serum is added to wells coated with purified antigen. IgG specific antibody, if present, binds to the antigen. All unbound materials are washed away and
epstein barr ebv test
ebv virus lab test
ebv testing history
$829.99
elisa test ebv|epstein barr ebv test